K Value Formula Chemistry

Updated february 03 2020.
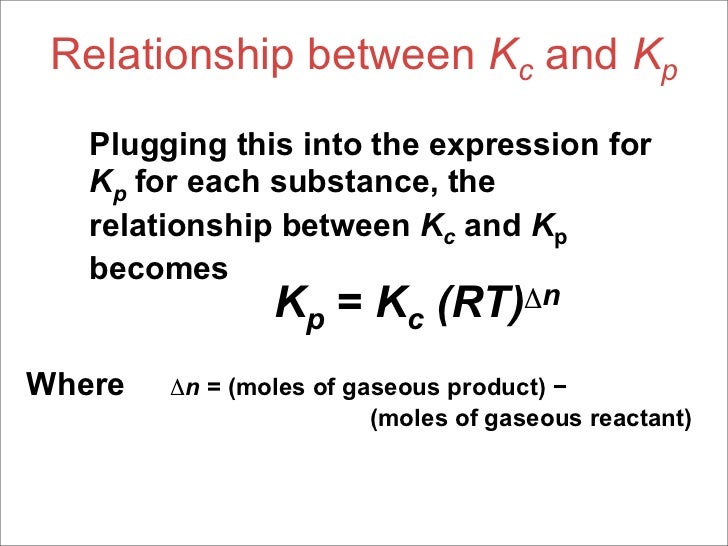
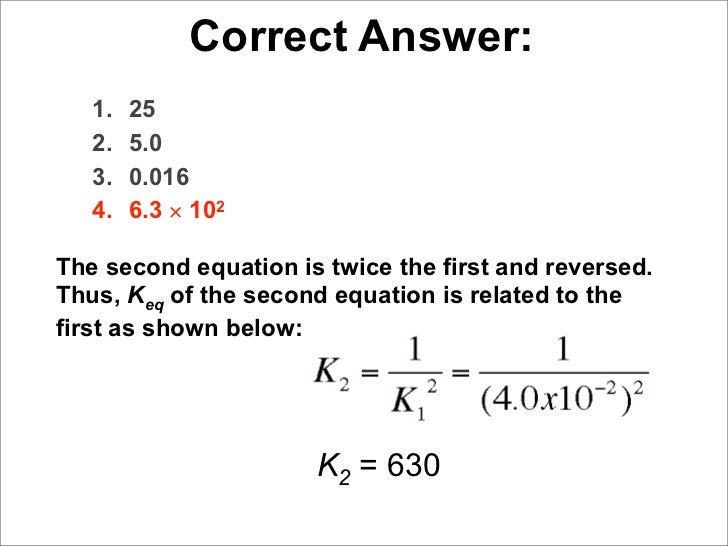
K value formula chemistry. C g the mass action expression for this reaction is. The value of k a is used to calculate the ph of weak acids. The time it takes for the concentration to drop to one half its current value during the course of the reaction. K a h 3 o ch 3 ch 2 co 2 ch 3 ch 2 co 2 h k a x 2 0 2 x k a 1 32 x 10 5 2 0 2 1 32 x 10 5 k a 8 69 x 10 10 ph pka ka pkb and kb explained.
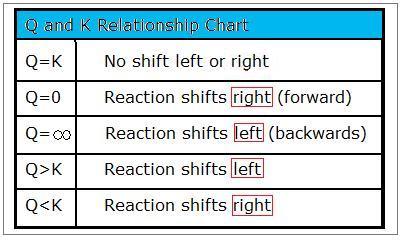
Plug in this value for x to solve for k a. A a products all the following equations assume that k is for the overall reaction. E k a k z a 2. And this cosntant is known as the equilibrium constant and is given the symbol k so at equilibrium.
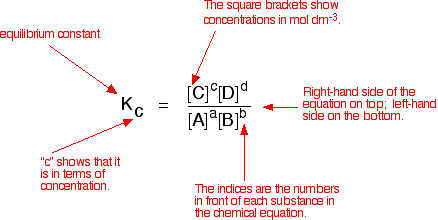
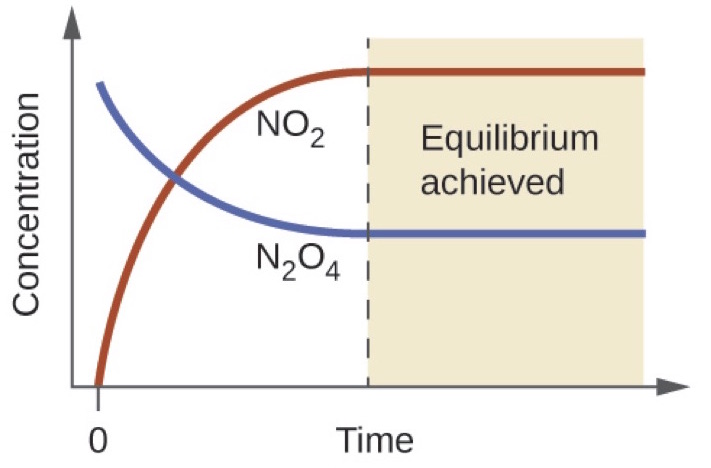
K a is the equilibrium constant for the dissociation reaction of a weak acid. The balance between the product and reactant of a chemical solution is called the equilibrium. C g a g b g at equilibrium the value of the mass expression is a constant that is q a constant. Definition formula equilibrium constant often denoted by k c is a numerical value that is derived from the ratio of the concentrations of the products to the concentrations of the reactants of a chemical solution at the state of equilibrium.
Displaystyle frac e k a equiv k frac z a 2 where. The pk a value is used to choose a buffer when needed.

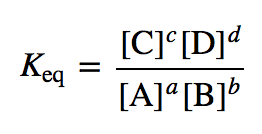








/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)