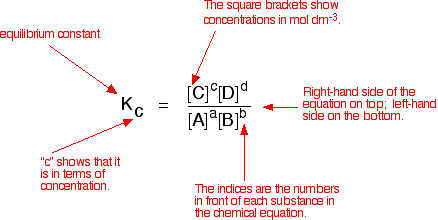
K Values Chemistry

The force constant of a spring see hooke s law.
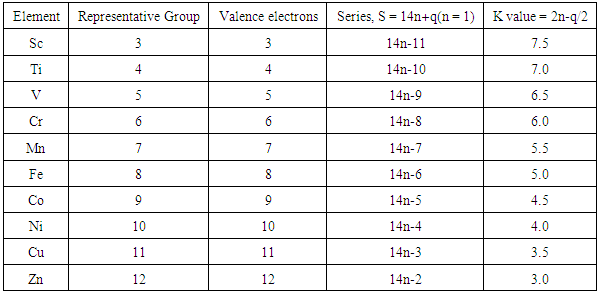
K values chemistry. K value or k value may refer to. Which of the following statements would be true. Analysis provides a methodology for studying different factors that affect the size of a biological population. Determine the relative value for k c at 100 o c.
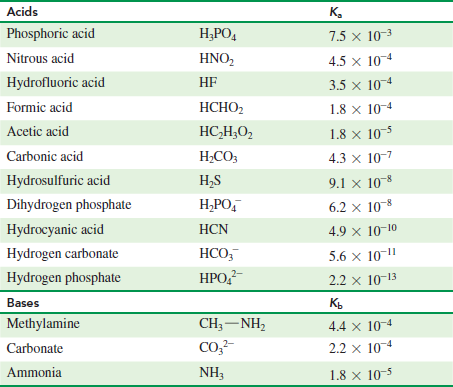
Reversible reactions equilibrium and the equilibrium constant k. An acid dissociation constant k a also known as acidity constant or acid ionization constant is a quantitative measure of the strength of an acid in solution it is the equilibrium constant for a chemical reaction known as dissociation in the context of acid base reactions the chemical species ha is an acid that dissociates into a the conjugate base of the. The reaction will proceed to the right. We also acknowledge previous national science foundation support under grant numbers 1246120 1525057 and 1413739.
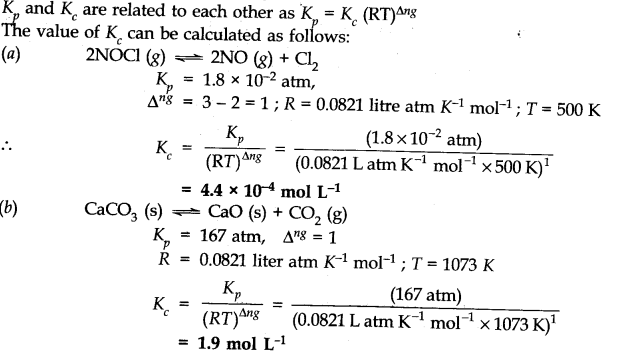
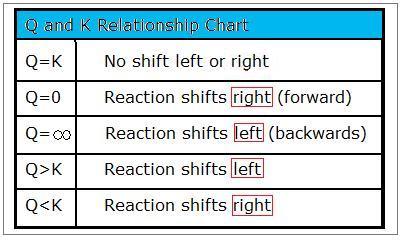
Q and k values chemistry. Formula to calculate numeric value of equilibrium constant of a solution. The libretexts libraries are powered by mindtouch and are supported by the department of education open textbook pilot project the uc davis office of the provost the uc davis library the california state university affordable learning solutions program and merlot. The relative permittivity κ.
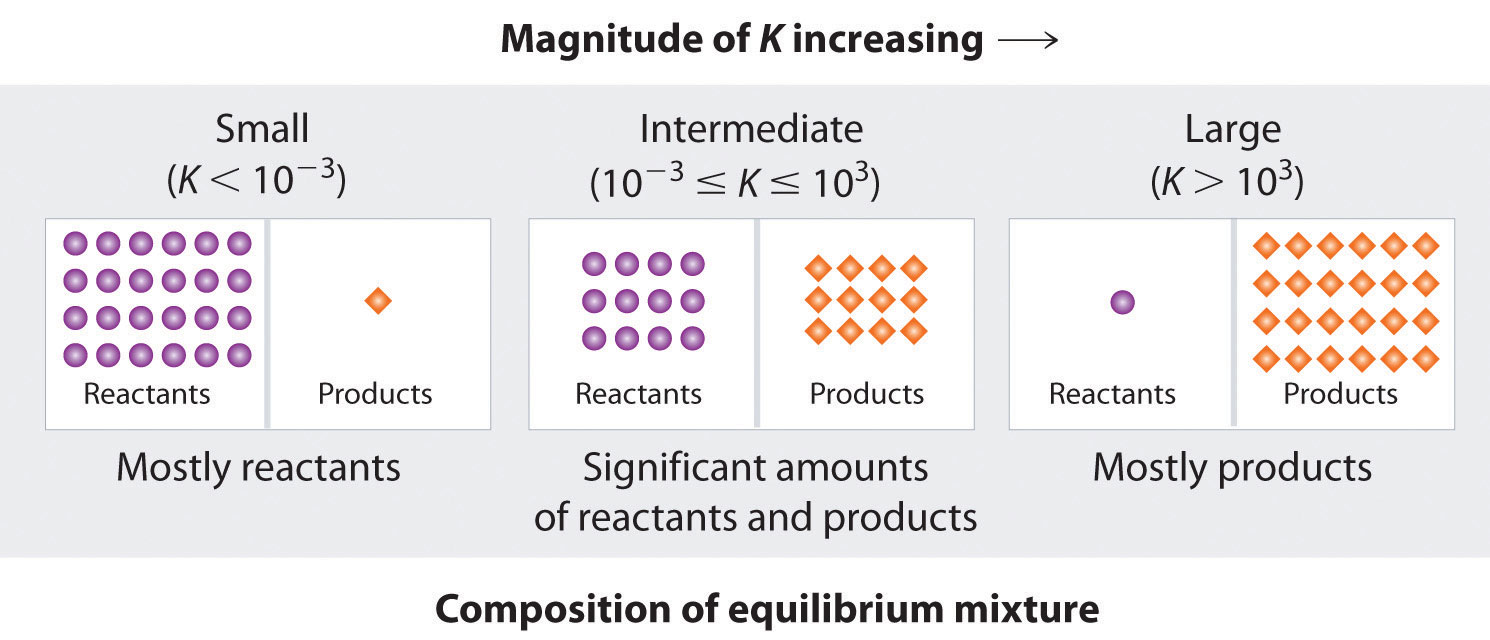
At a particular point in time a given reaction is found to have a k value larger than 1 and a q value less than 1. The reaction is already at equilibrium. 100 c is a higher temperature than 25 c therefore k c for this endothermic reaction will increase. Is your answer plausible.
Pause to ponder plausibility. To solve the problem first write the chemical equation for the reaction. Vapor liquid equilibrium the ratio of vapor concentration to liquid concentration at equilibrium. A statistical value used in the elo rating system.
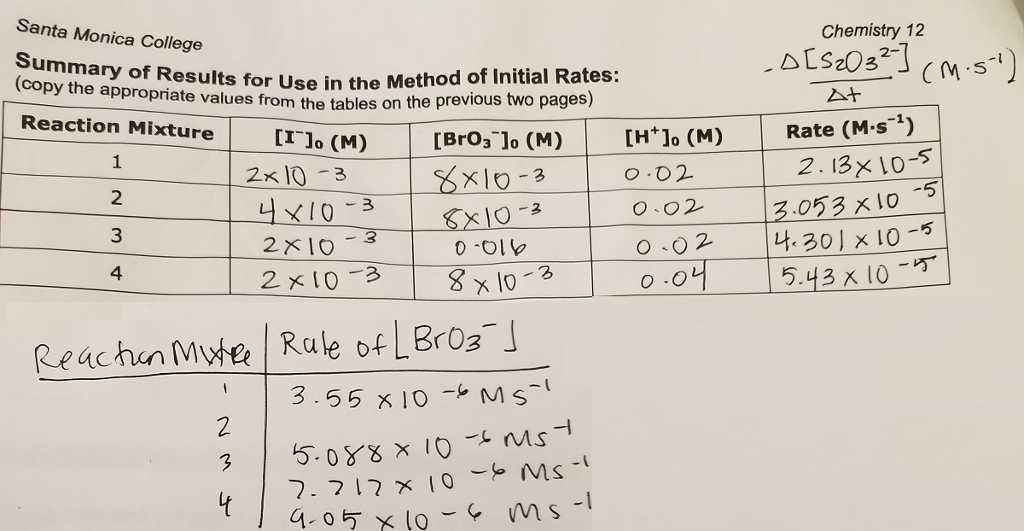
How to calculate k and how to use k to determine if a reaction strongly favors products or reactants at equilibrium.


/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)