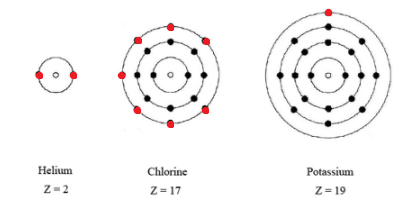
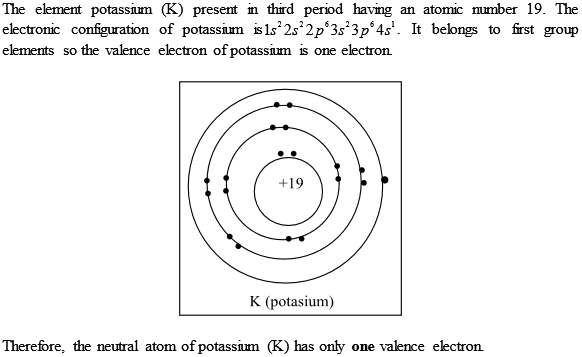
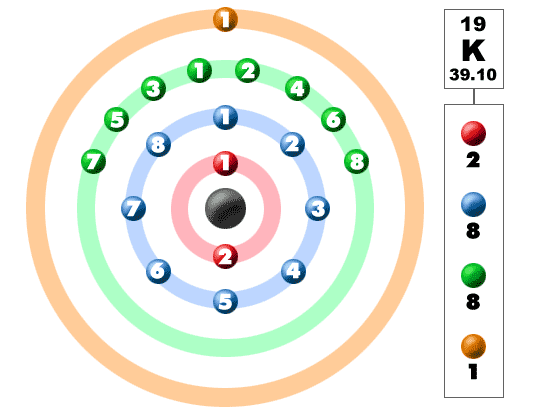
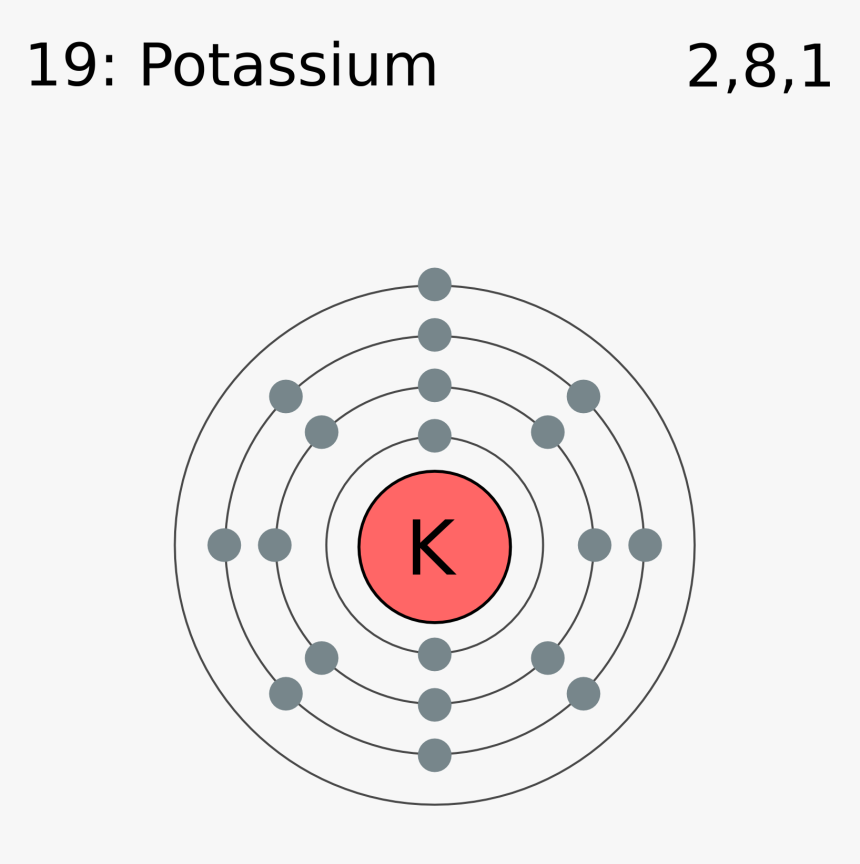
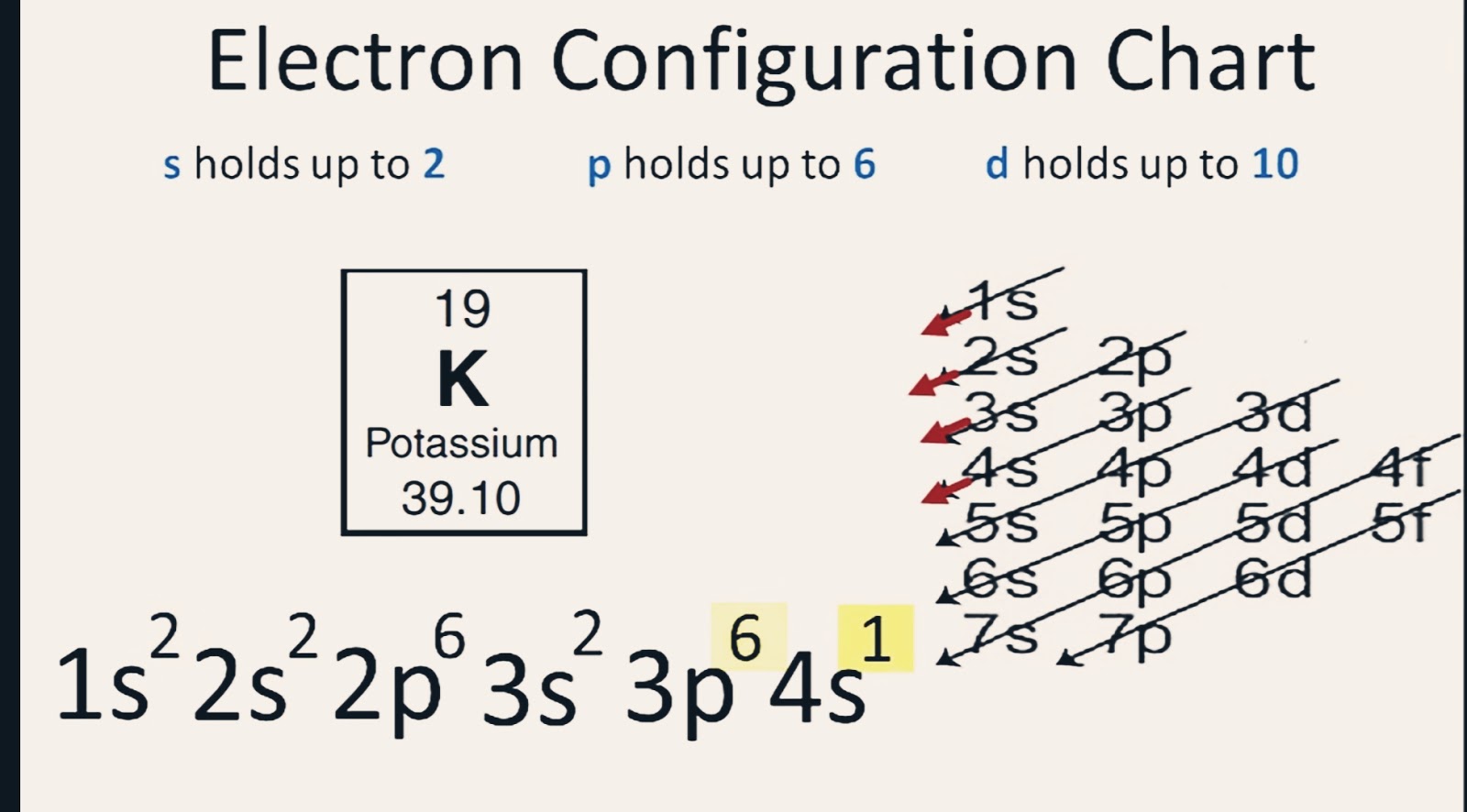
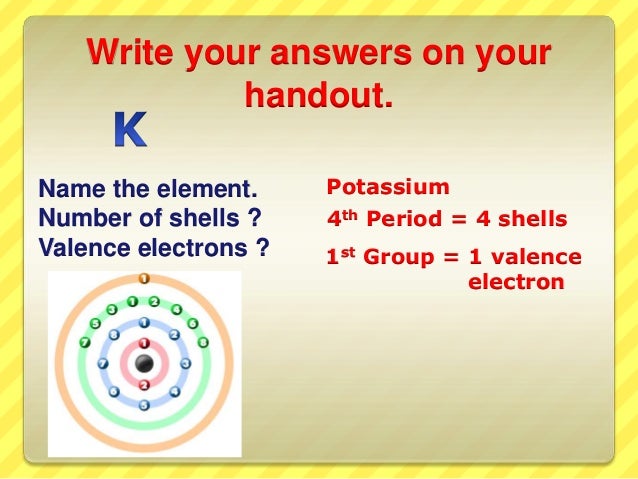
K Valence Electrons

This is because such an atom has only a single valence electron.
K valence electrons. The most reactive kind of metallic element is an alkali metal of group 1 e g sodium or potassium. Whether it is electronegative or electropositive in nature or they indicate the bond order of a chemical compound the number of bonds that can be formed between two atoms. Knowing how to find the number of valence electrons in a particular atom is an important skill for chemists because this information determines the kinds of chemical bonds that it can form and therefore the element s reactivity. Potassium s name in other languages.
The number of valence electrons in an atom governs its bonding behavior. The electron configuration for k 42 is 2 8 8 1 meaning that there is one electron in the outermost shell of the atom. Also as saltpeter potassium nitrate kno 3 it is used to make explosives and to color fireworks in mauve. In chemistry valence electrons are the electrons that are located in the outermost electron shell of an element.
Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Used as potash in making glass soap lenses and salt substitute. Therefore elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements. Valence electrons of all the elements in the periodic table in graph and table format complete information about all the properties of elements using graphs and tables interactive dynamic periodic table periodic table element comparison element property trends and complete information about the element facts how to locate on periodic table history abundance physical properties.
Valence electrons are of crucial importance because they lend deep insight into an element s chemical properties.