K Values Chemistry Table

Fec 2 o 4.
K values chemistry table. K a dfrac x 2 0 150 x 1 6 times 10 2. Sio 2 s quartz 910 94. The relative permittivity κ. Cuc 2 o 4.
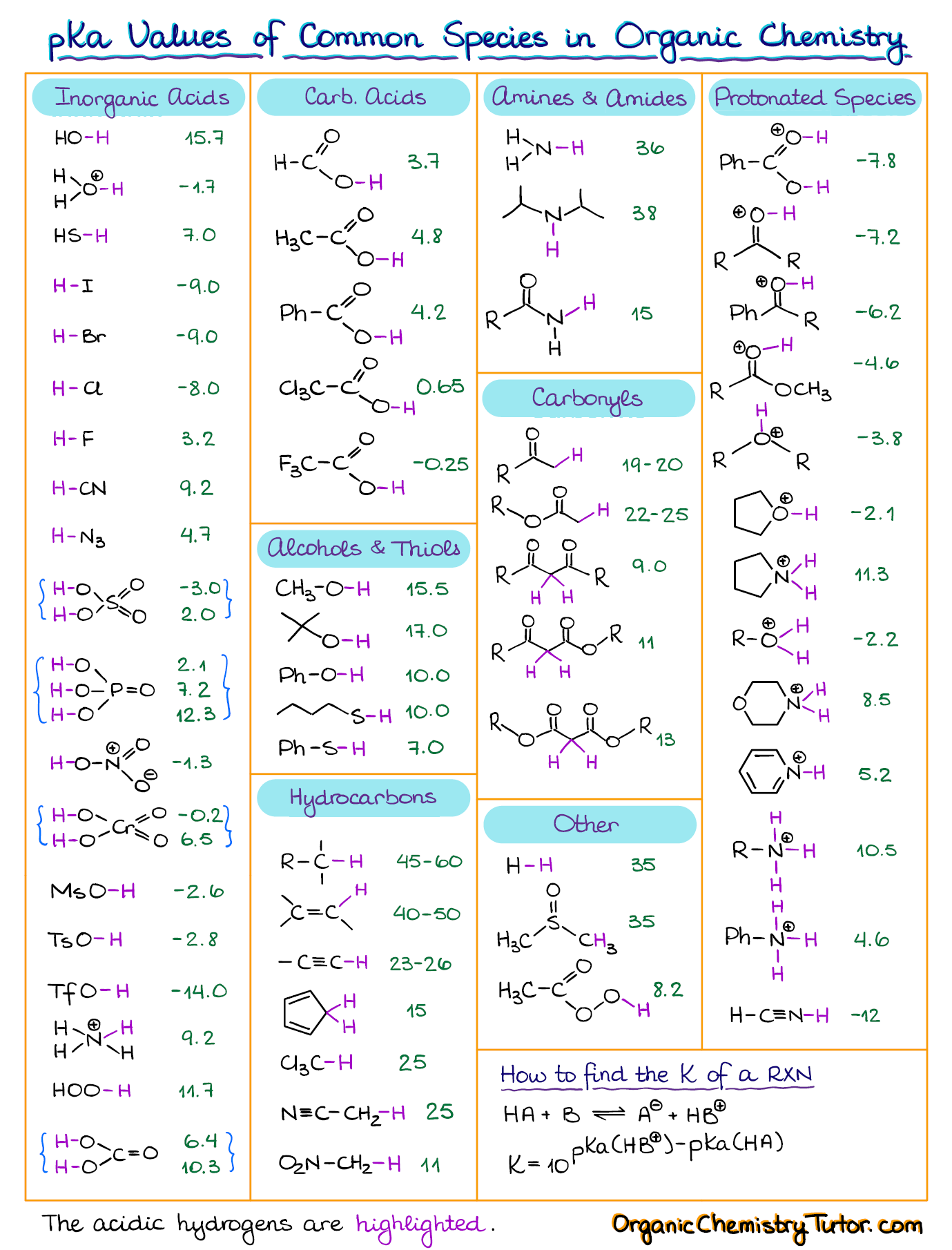
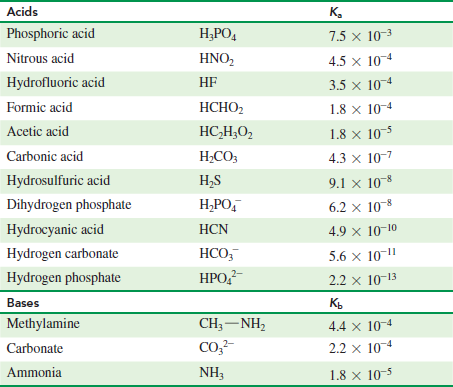
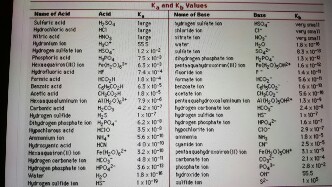
Acid with values less than one are considered weak. Values for electronegativity run from 0 to 4. Molarity molality and normality. Mgc 2 o 4.
Aa g bb g cc g. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Nic 2 o 4. Note that the same r i c e.
Src 2 o 4. Analysis provides a methodology for studying different factors that affect the size of a biological population. Choosing an acid or base where pk a is close to the ph needed gives the best results. Sih 4 g 34 3.
And the values for the concentration of each species at equilibrium that is the values of a eq b eq and c eq found using the r i c e. K value or k value may refer to. Predict the value for an equilibrium constant k at a different temperature. A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
The value of k a is used to calculate the ph of weak acids the pk a value is used to choose a buffer when needed. Use the ice table to calculate concentrations with k a the expression for k a is written by dividing the concentrations of the products by the concentrations of the reactants. Anatomy of the atom answers many questions regarding the structure of atoms. Bac 2 o 4.
Cdc 2 o 4. It can also be used to predict if the resulting molecule will be polar or nonpolar. N 2 o 4 g 2no 2 g at 25 c the value of the equilibrium constant k c is 4 7 10 3. This table is a list of electronegativity values of the elements.
Strong acids are listed at the top left hand corner of the table and have ka values 1 2. Vapor liquid equilibrium the ratio of vapor concentration to liquid concentration at equilibrium. The force constant of a spring see hooke s law. A statistical value used in the elo rating system.
K a is the equilibrium constant for the dissociation reaction of a weak acid a weak acid is one that only partially dissociates in water or an aqueous solution. Hg 2 br 2. The strong bases are listed at the bottom right of the table and get weaker as we move to the top of the table.










/what-is-pka-in-chemistry-605521_FINAL2-9fdfc39e9aa34caa96d6e74a2c687707.png)