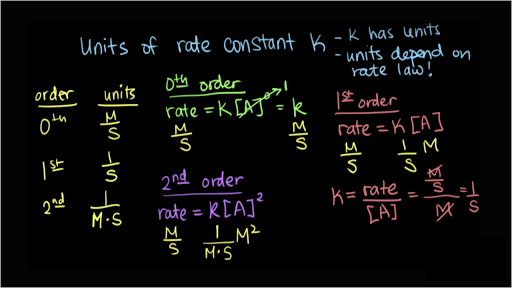K Value Units Chemistry

So these are the three most common molecularities that you might see in a chemistry class.
K value units chemistry. The equilibrium constant of a chemical reaction is the value of its reaction quotient at chemical equilibrium a state approached by a dynamic chemical system after sufficient time has elapsed at which its composition has no measurable tendency towards further change for a given set of reaction conditions the equilibrium constant is independent of the initial analytical concentrations of the. Ln ph2 k t ln ph2 where the ln ph2 on the left hand side of the equal sign is the concentration of phenolphthalein at any time t and the ln ph2 on the right hand side of the equal sign is the initial concentration. Here k t is the reaction rate constant that depends on temperature and a and b are the molar concentrations of. In chemical kinetics a reaction rate constant or reaction rate coefficient k quantifies the rate and direction of a chemical reaction.
Display the exponent from a binary floating point number as a decimal value spiral. Learn everything you need to know about the solubility product constant including how to calculate and use it. Calculate the acid dissociation constant k a for a 0 2 m aqueous solution of propionic acid ch 3 ch 2 co 2 h that is found to have a ph value of 4 88. Confused about ksp chemistry equations.
Formula to calculate numeric value of equilibrium constant of a solution. To solve the problem first write the chemical equation for the reaction. The k s value is divided by the molar concentration of the solvent so that n p o t is dimensionless. Reversible reactions equilibrium and the equilibrium constant k.
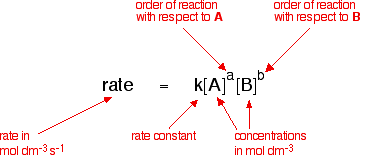
For a reaction between reactants a and b to form product c a a b b c c. And sometimes you have reactions that aren t zeroth first or second order and whenever that happens you can always use the rate law to find the units of the rate constant k. In the np t scale no such correction is applied and np t has the units m 1. The reaction rate is often found to have the form.
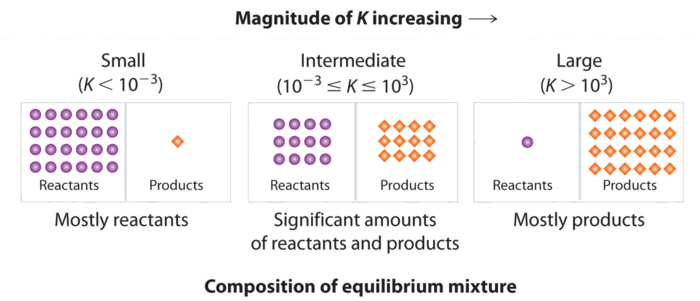
How to calculate k and how to use k to determine if a reaction strongly favors products or reactants at equilibrium. Chemistry stack exchange is a question and answer site for scientists academics teachers. Equilibrium constant often denoted by k c is a numerical value that is derived from the ratio of the concentrations of the products to the concentrations of the reactants of a chemical solution at the state of equilibrium the balance between the product and reactant of a chemical solution is called the equilibrium. Lavery in theoretical and computational chemistry.
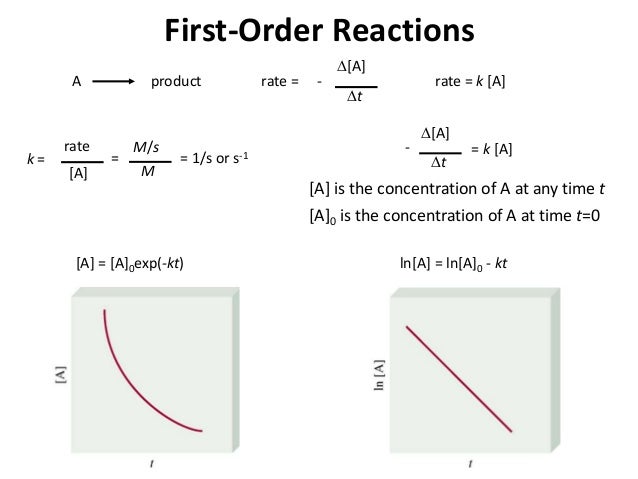
If i was asked the units of k in the first order rate equation shown below.