K Number Of Valence Electrons

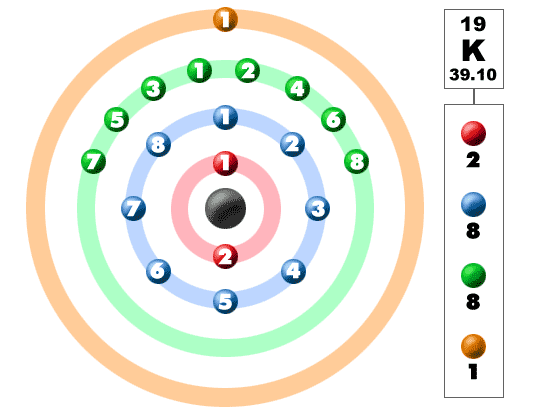
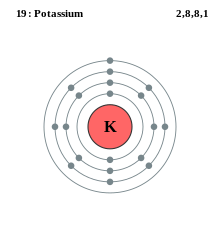
When we write the configuration we ll put all 19 electrons in orbitals around the nucleus of the potassium atom.
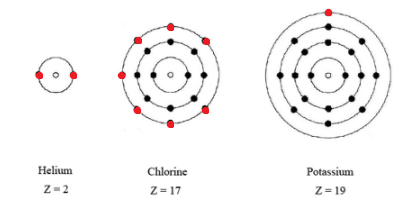
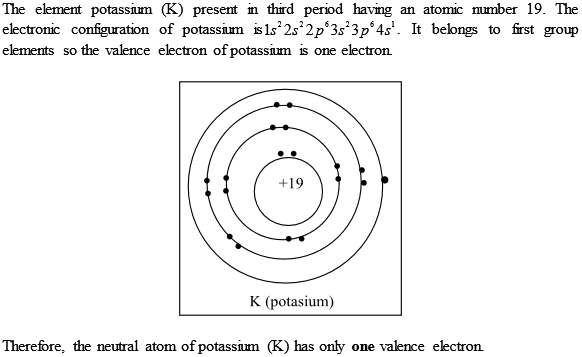
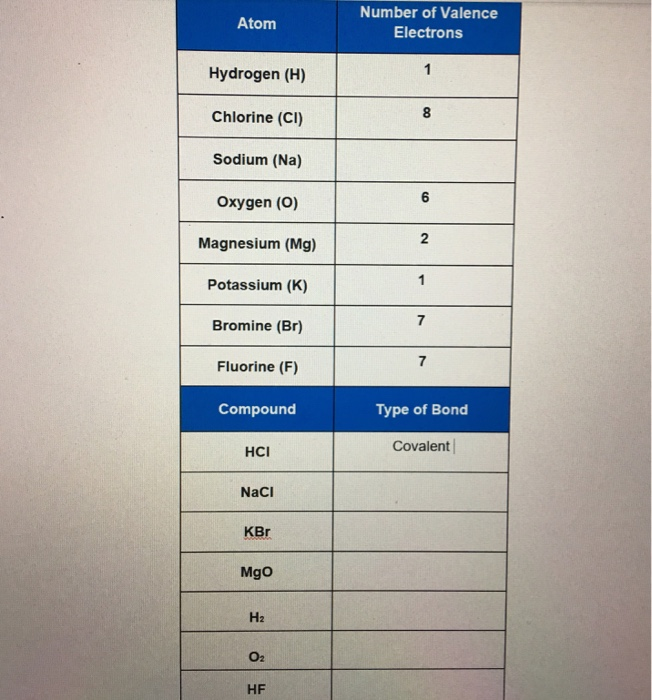
K number of valence electrons. Click on element atomic number element symbol element name and element valence electrons headers to sort. Potassium k has 19 total electrons as indicated by its atomic number the number of electrons equals the number of protons in a neutral atom. The 4th energy level no longer exists. The number of valence electrons is now 1.
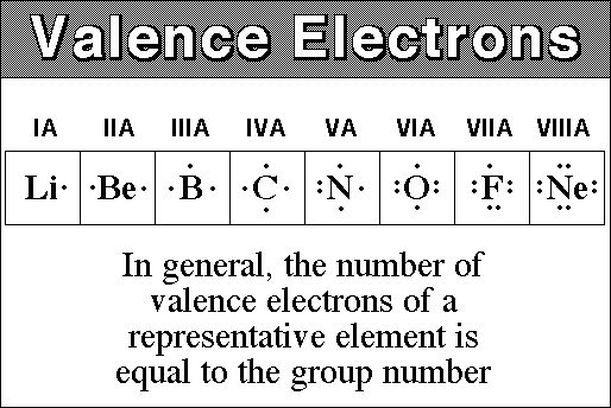
Valence electrons chart valence electrons of all the elements in table chart. The most reactive kind of metallic element is an alkali metal of group 1 e g sodium or potassium. In writing the electron configuration for potassium the first two electrons will go in the. Electrons per energy level.
Therefore elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements. This is also observed in the inner transition elements due to the comparable energy levels of f d and s shells. Number of neutrons most common stable nuclide. If k loses the 4s 1 electron it will have 8 valence electrons a full octet in the 3rd energy level.
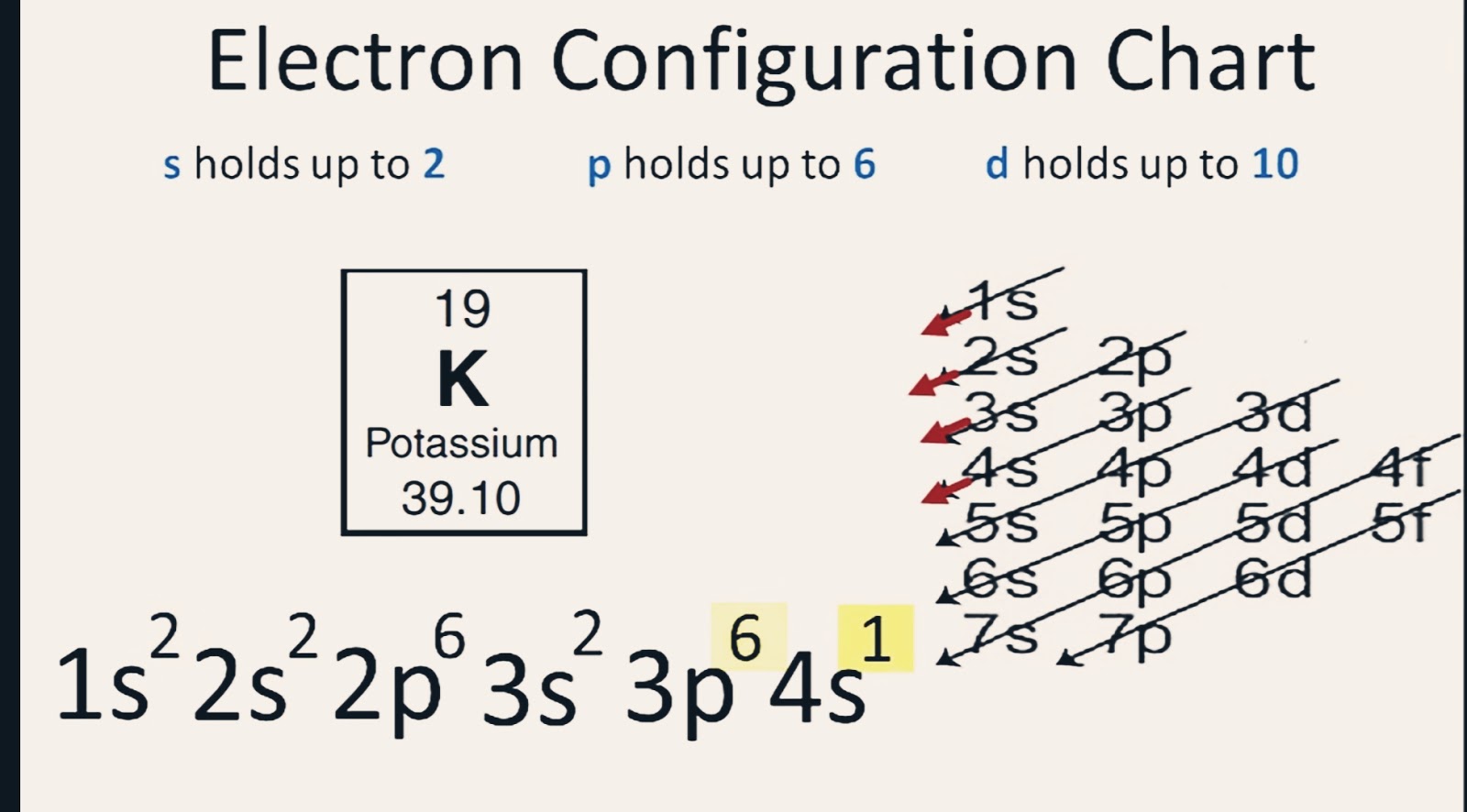
This valence electrons chart table gives the valence electrons of all the elements of periodic table. In order to write the potassium electron configuration we first need to know the number of electrons for the k atom there are 19 electrons. 1s 2 2s 2 p 6 3s 2 p 6 4s 1. 4s 1 electron dot model.
1s 2 2s 2 2p 6 3s 2 3p 6 full octet in 3s 2 3p 6 3 having a full octet in the highest energy level will make the ion atom that has gained or lost electrons energetically stable explained through quantum mechanics and thermodynamics 4 if k 1. The number of valence electrons in an atom governs its bonding behavior. Number of electrons with no charge.