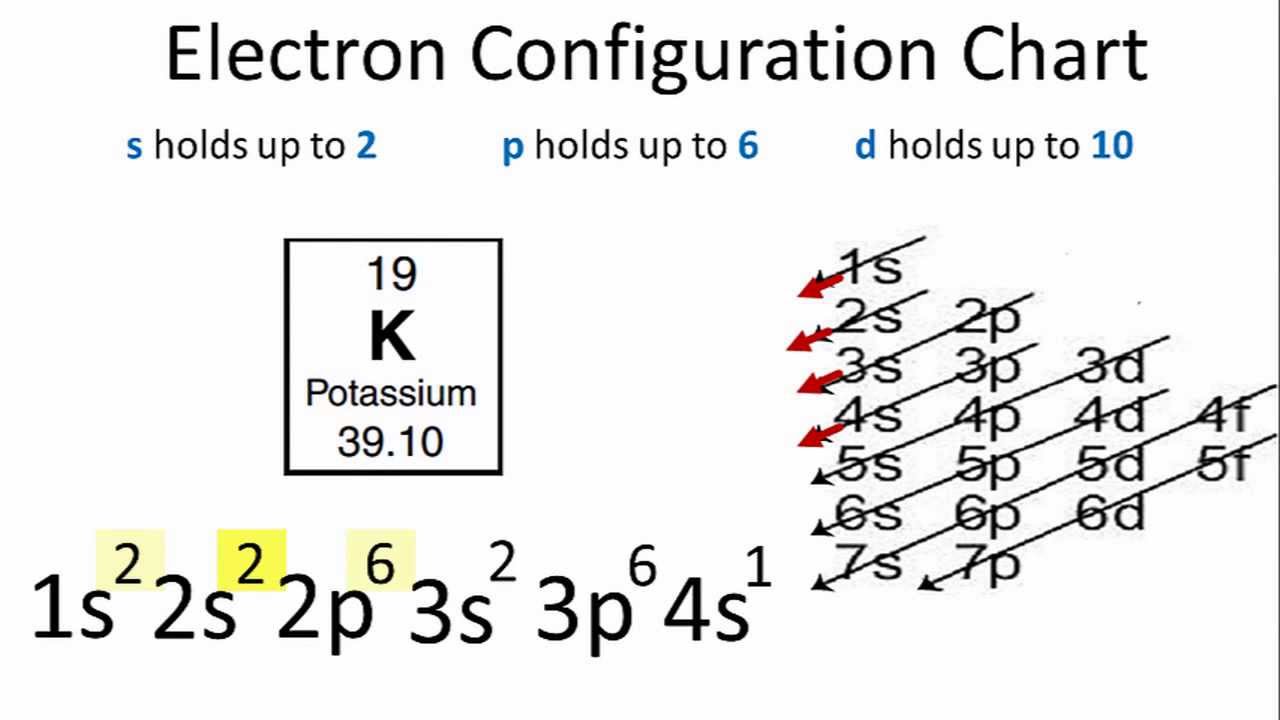
K Element Valence Electrons
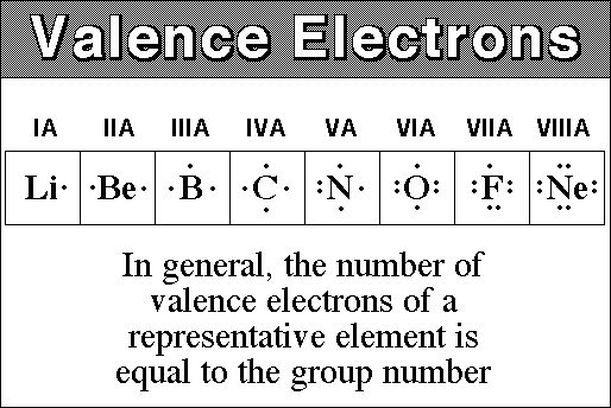
In the below periodic table you can see the trend of valence electrons.
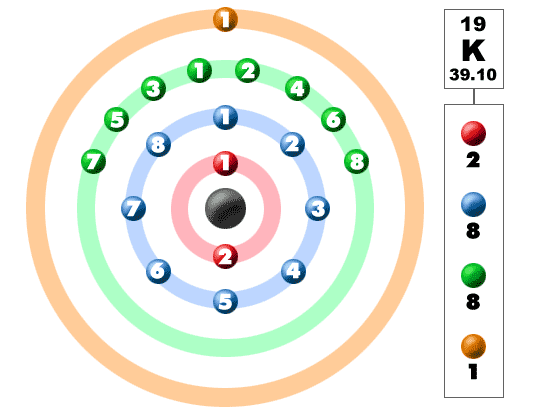
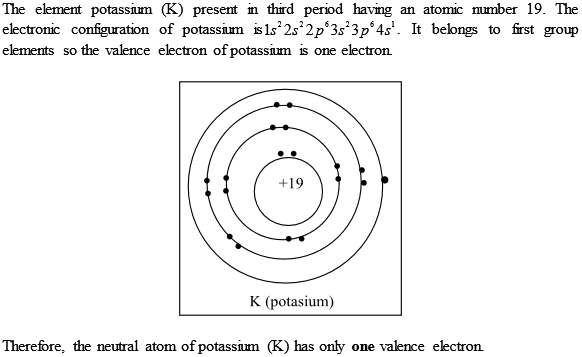
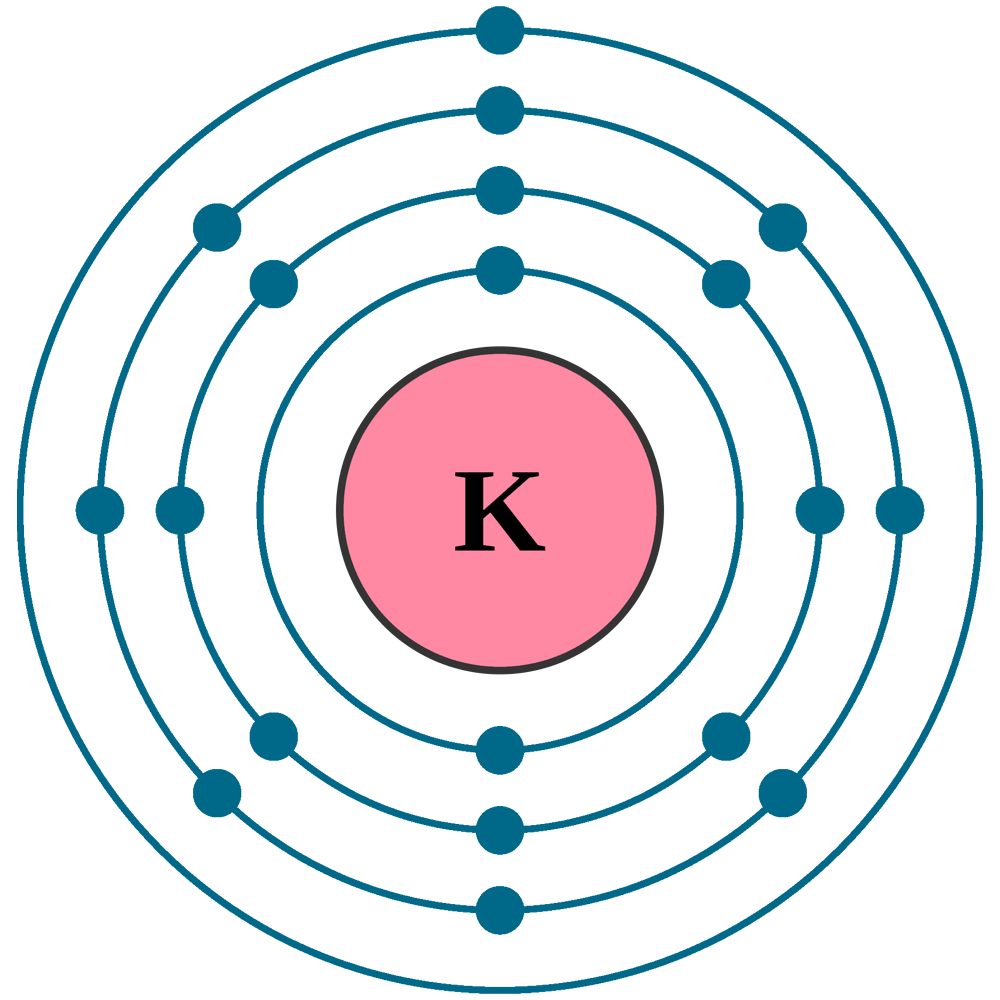
K element valence electrons. Whether it is electronegative or electropositive in nature or they indicate the bond order of a chemical compound the number of bonds that can be formed between two atoms. This is because such an atom has only a single valence electron. Periodic table of elements with valence electrons trends. The number of valence electrons is what determined the reactivity of an atom.
The number of valence electrons in an atom governs its bonding behavior. For facts physical properties chemical properties structure and atomic properties of the specific element click on the element symbol in the below periodic table. Remember that an element s electron cloud will become more stable by filling emptying or half filling the shell. Including scores of properties element names in many languages most known nuclides of potassium.
Also shells don t stack neatly one on top of another so don t always assume an element s valence is determined by the number of electrons in its outer shell. Common chemical compounds are also provided for many elements. K is the symbol for potassium and the number of valence electron can be found through its group on the periodic table. The most reactive kind of metallic element is an alkali metal of group 1 e g sodium or potassium.
Comprehensive data on the chemical element potassium is provided on this page. Therefore elements whose atoms can have the same number of valence electrons are grouped together in the periodic table of the elements. Here is a table of element valences. Periodic table of elements element potassium k.
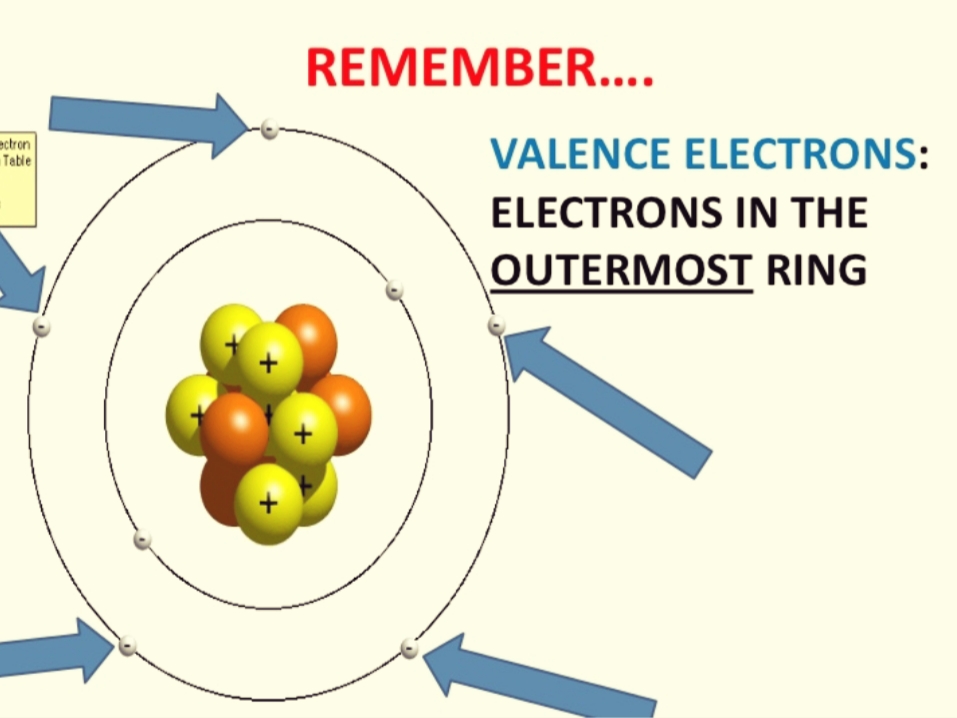
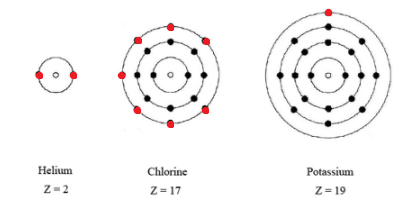
Valence electrons are electrons in the outer energy level of an atom that interacts with other atoms.